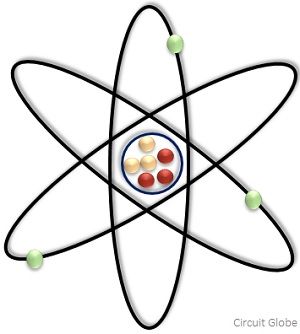
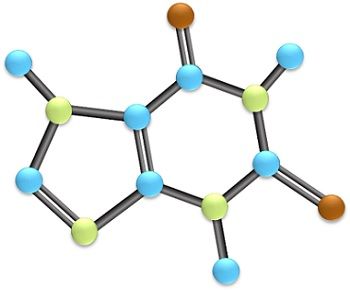
Atoms and Molecules are the two most basic terms used whenever we talk about the composition of an element or compound. The significant difference between atom and molecule is that an atom is regarded as the tiniest particle that constitutes matter. On the contrary, a molecule is the combination of two or even more smallest units i.e., atoms that are chemically bonded together.
Whenever the names atoms and molecules are taken, there occurs confusion that how the two are different and what similarities exist between the two.
Basically, something which requires a definite space and has some specific mass is defined as matter, and the matter is composed of atoms and molecules. Everything in this universe is composed of atoms. From a small piece of chalk to a large blackboard, from a single wall to a complete home, from a small ant to a giant human, and from a giant human to this whole earth, everything is composed of atoms. But atoms are such small entities that it is impossible to visualize them through human vision or any microscopic device.
Content: Atom Vs Molecule
Comparison Chart
| Basis for Comparison | Atom | Molecule |
|---|---|---|
| Basic | The smallest indivisible unit of matter is atom. | A combination of multiple atoms which can be further divided is molecule. |
| Existence | Not independent | Independent |
| Components | Nucleons (having protons, neutrons) and electrons. | Two or more atoms bonded together. |
| Nature | Unstable | Stable |
| Visibility | No | Yes, but only through microscope |
| Mass | Approximately equals to sum of masses of protons, neutrons and electrons consisting that atom. | Sum of masses of each individual atom forming the molecule. |
| Shape | No fixed shape but generally assumed to be spherical. | Exists in linear, trigonal, pyramidal shapes, etc. |
| Reactivity | High | Low |
| Size | Small | Comparatively large |
| Bonding | Coulomb’s force of attraction binds the subatomic particles. | Covalent bond exists between two or more atoms. |
| Example | Sulphur, Nitrogen, Sodium, Carbon, etc. | Water, Sugar, Carbon Dioxide, hydrogen peroxide, etc. |
Definition of Atom
The smallest particle, indivisible in nature that constitutes the matter is known as an atom. Each atom has three major subatomic constituents namely, electron, proton, and neutron. And each atom is different from one another because they have different number of protons.
Atoms are regarded as the basic building block of any matter and show their existence everywhere around us.
Basically, the theory behind this is according to Maharishi Kanad, on dividing any matter, smaller and smaller particles will be obtained. But after a certain point of time, the particles will be this much small that their further division will not be possible. This ultimate undividable particle is referred as an atom.
The word atom is derived from a combination of words ‘A’ and ‘tomas’ where ‘A’ means ‘not’ and ‘tomas’ corresponds to ‘cut’.
Each atom exhibits the properties of the chemical element in which it is present. The atomic number is a crucial property of an atom that corresponds to the number of positive charges existing in the nucleus.
Definition of Molecule
A chemical combination of multiple indivisible smallest units of matter i.e., atoms generates a molecule. Whenever two or more than that atoms tightly bonded together by some force of attraction then their individual combination produces a molecule. A molecule is referred as that smallest entity of a matter that exhibits independent existence, can be divided further, and displays the characteristics of the material matter.
The combination of atoms that form a molecule can be of the same or different elements.
On general basis molecules are classified as:
Molecules of Elements: These are the molecules formed by a combination of similar type of atoms.
Example – Oxygen (O2) is a molecule composed of 2 separate oxygen atoms thus is a diatomic molecule.
Molecules of Compounds: These are the molecules that are composed of atoms of different types of elements.
Example – Calcium Oxide (CaO) is a molecule that is a combination of carbon and oxygen atom.
Various atoms in a molecule are bonded together by sharing the electrons present in their outer shells through covalent bonding.
Key Differences Between Atom and Molecule
- The key factor of differentiation between atoms and molecules lies in their presence as individual entities. This is so because the atom as an individual is the smallest unit of the matter which cannot be divided further. As against, a molecule as an individual is the combination of two or even more atom that exists as the smallest unit of an element which is identifiable in nature and can be divided further.
- The composition of an atom is the nucleus (consisting of positively charged protons and neutrally charged neutrons) which is surrounded by electrons that are negatively charged. While the molecules are composed of two or even more atoms that are combined together and exhibit the properties possessed by the atoms that form it.
- Most of the atoms do not exist independently however, molecules show independent existence.
- Atoms are single entities but the subatomic particles are bonded together by coulomb’s force of attraction existing between positively and negatively charged particles. However, to form a molecule various atoms are combined together through a covalent bond.
- Atoms are highly reactive in nature in comparison to molecules.
- Atom exhibits unstable behavior because of the presence of electrons in the outer shells. On the contrary, as atoms within the molecules are combined through covalent bonds thus exhibit stable nature.
- Atoms are the tiniest particles of matter thus can neither be seen through naked eyes nor through a microscope. But though molecules can also be not seen through naked eyes, can be seen using a magnifying microscope.
- Obviously, as molecules are a combination of atoms thus their size will be more than the size of an atom. Generally, the diameter of an atom is around 0.2 nm while the size of a water molecule is nearly 0.27 nm.
- Generally, it is known that atoms are spherical in shape but in actuality, no fixed shape is possessed by atoms. While the shape of molecules can be linear, tetrahedral, pyramidal, trigonal, etc. depending on the atomic composition of that molecule.
Conclusion
So, from this discussion, we can conclude that atoms and molecules are similar in the fact that both constitute matter. However, atoms cannot be further divided, some examples of atoms are nitrogen, sulphur, carbon, etc. And molecules can be further divided whose division will provide us atoms showing the characteristics of the substance, some examples of molecules are water, carbon dioxide, etc.