Electrons and Protons are two prime components of an atom out of three. The crucial difference between electron and proton is that an electron is a charged particle with negative polarity. As against, a proton is a charged particle having a positive charge. Both electron and proton are the fundamental components of an atomic structure and has their own significance.
What is an atom?
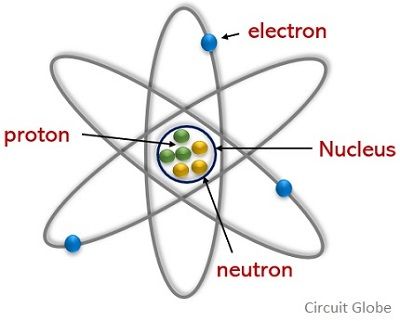
We know that an atom is regarded as the smallest particle as it is the fundamental unit by which a matter is composed. While this atom itself has 3 major subatomic particles which are known as electron, proton, and neutron.
Multiple atoms form a molecule and the atoms inside a molecule are attached by chemical bonds. The electric charge of the atom maintains the bond between the atoms in a molecule. Among electron, proton, and neutron, the electrons and protons are negatively and positively charged respectively while the neutrons are neutrally charged particles.
Electrons and protons hold different properties and are present at different locations inside the atom. Therefore, has various differentiating factors which we will discuss here.
Content: Electron Vs Proton
Comparison Chart
| Basis for Comparison | Electron | Proton |
|---|---|---|
| Symbolized as | e | p |
| Polarity | Negative | Positive |
| Location within the atom | Outside the nucleus in a well-defined orbit. | Present inside the nucleus. |
| Electric charge | -1 | +1 |
| Mass | Less (9.1 * 10-31 Kg) | Comparatively more (1.67 * 10-27 kg) |
| Moving ability | Exist | Not exist |
| Removal or addition | These can be easily removed from or added to an atom. | These are very difficult to get added or removed. |
Definition of Electron
A subatomic particle of an atom that holds an electric charge of negative polarity. Inside an atom, ideally, electrons are present in spherical shells and move around the nucleus in an orbital path. While when external energy is supplied to the electrons then they move from an atom to another.
Basically, the energy supplied to the electrons frees them from the shells thus they become mobile and get attached to its nearby atom whenever there is a deficiency of electron in that particular atom.
In the case of conductors, the movement of electrons is the reason for the flow of current. And an electron is considered to have a unit electrical charge generally represented as e. The electron charge is measured in Coulomb and has a value of about 1.602 * 10-19 C. And all the electrons are considered to be identically similar to each other.
Definition of Proton
Proton is another major particle of an atom with a charge of positive polarity. It’s a crucial component of an atom that forms the nucleus of an atom with the neutron. As the nucleus of the atom is at the center thus a proton holding a positive charge is present at the center of the atomic structure.
It is denoted by a symbol p and has a charge +1.
A noteworthy point over here is that the number of protons that are present in an atom signifies its atomic number. As the nucleus holds both protons and neutrons thus combinedly called nucleons. As electrons and protons hold the same charge of opposite polarity thus there exists a force of attraction between them inside the atom. Due to this attractive force, the electrons and protons are attached within the atom.
This is the reason ideally the electrons are bounded and moves in the orbital path. An atom has the same number of electrons and protons so the positive and negative charges get canceled making the atom electrically neutral.
Key Differences Between Electron and Proton
- An electron is a negatively charged component of an atom whereas the proton is a positively charged body.
- The electrons are present outside the nucleus in the orbiting shells. But the protons along with neutrons form the nucleus of the atom and are present at the center of the atomic nuclei.
- Electrons are highly mobile as these are present in the shells of the atoms and can be easily freed on supplying energy. However, as the proton is present at the nucleus of the atom thus is not mobile like the electrons.
- The polarity of electrons is negative while that of the proton is positive.
- The mass of a proton is 2000 times of an electron thus is quite higher than the mass of the electron. Generally, the mass of an electron is 9.1 * 10-31 Kg while that of the proton is 1.67 * 10-27 kg.
- The addition and removal of electrons to or from the atom are quite easy due to presence in the orbits. While adding and removing the protons is not an easy task.
Conclusion
Thus, this discussion concludes that all the electron, proton, and neutron constitute an atom but as the neutron is neutrally charged thus the force of attraction between two unlike charges i.e., between electron and proton binds the subatomic particles within the atom.

great article