Both charge and mass are the properties of matter. The significant difference between charge and mass is that charge has a classification. As against, the mass has no further classification. Charge mainly exists in two types positive and negative but mass can never be negative, it is always positive.
A charge is a component that contributes to electricity. On the contrary, mass is a component that contributes to gravitation.
We will discuss the various factors over which charge can be distinguished from the mass.
Content: Charge Vs Mass
Comparison chart
| Basis for Comparison | Charge | Mass |
|---|---|---|
| Basic | It produces electric field. | It produces gravitation field. |
| Classification | Positive and negative | Always positive |
| Denoted as | q | m |
| Nature | Quantized | Non-quantized |
| SI unit | Coulombs | Grams or Kilograms |
| Existing force | Attractive or repulsive | Attractive only |
| Conservation | Exist | Not exist |
| Dependency on speed | Independent | Dependent |
| Existence | Cannot exist without mass | Can exist without charge |
Definition of Charge
A charge is defined as a property of matter. It is denoted by q and classified into two categories namely positive and negative. We know that atom is the smallest individual element that constitutes a matter. Also, according to the classification of charge, we can define charge as electron and proton. Basically, the property with which the two types of charges are differentiated is known as the polarity of charges.
The charges of the same polarity always repel each other as against charges of different polarities always exhibit attractive force. Sometimes we come across the word ‘point charge’, point charge corresponds to such charged bodies which are very small in size in comparison to the separation distance of charges.
Fundamental Properties of Electric Charge
- Additivity: For a system with ‘n’ individual charges, the total charge of the system will be the summation of each individual charge present within the system.
- Conservation: The total charge within a system is of conserved nature. This means that they may get redistributed i.e., can be transferred from one body to another but cannot be destroyed or created.
- Quantized: Charge always exists as an integral multiple of e. The charge on any body is given as:
q = ne
: e corresponds to the charge on an electron or proton.
Definition of Mass
Mass is known to be a fundamental property of matter. The mass of a body is the measure of the amount or density of atoms that a body consists of. Also, this measurement is independent of the place where the body is present and the applied gravitational field. Generally, mass is regarded as an unchangeable property of matter but with an increase in speed, mass of the moving body also increases. The relation between mass and moving velocity of an object is given as:
: m is the mass of moving body,
mo is the mass of the stationary body,
c is the speed of light 3 * 108 m/s
The mass of a body remains independent of the way particles is rearranged within the body.
Sometimes people get confused between the words mass and weight and use the two interchangeably. However, the two are different and there are various factors that differentiate mass and weight.
Key Differences Between Charge and Mass
- Electric charge or simply charge is a physical quantity that can be either positive or negative. The positive charge is called proton whereas the negative charge is known as the electron. While mass is a physical quantity that is always positive.
- Charge exhibits the property of quantization whereas mass is Non-quantized in nature.
- A charge may not exist without mass whereas mass can exist without net charge also.
- When the charge is transferred then a microscopic change in mass occurs. However, when mass is transferred then it does not bring change in charge.
- Charge on a body is independent of the velocity with which the body is moving. This implies charge shows invariable nature. As against, the mass of the body increases with the increase in moving velocity of the body. This is justified by the relation:
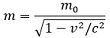
- A charge is a derived physical quantity while mass is a fundamental physical quantity.
- Force existing between charges can be either attractive or repulsive depending on the polarity of charges. Whereas only an attractive type of force exists between massive bodies.
- The measuring unit of charge is Coulomb while mass is measured in either grams or kilograms.
Similarities
Both charge and mass are known to be the properties of matter. Charge and mass can be added as real numbers, both have magnitude and no direction thus are scalar quantities.
Conclusion
Proton and electron are the two types of charges let us see what charge and mass both proton and electron hold. Proton is positive and has a charge of the magnitude of +1.6 * 10-19 C and a mass of 1.67 * 10-27. While electron is negative with a charge of magnitude -1.6 * 10-19 C and mass of 9.11 * 10-31.
This means the values of charge and mass are different.
